
Delivering on the promise of RNA therapeutics to defeat cancer
TransCode Therapeutics is an RNA oncology company, created on the belief that cancer can be defeated through the intelligent design and effective delivery of RNA therapeutics.
For decades, RNA has been a topic of investigation by the scientific community as a potentially attractive therapeutic modality because it can target any gene and it lends itself to rational and straightforward drug design. RNA-based therapeutics are highly selective to their targets with the potential to make available a broad array of previously undruggable targets in the human genome. To date, use of RNA for therapy has been limited due to three delivery-related challenges: protecting the RNA from being dismantled by the immune system; maintaining stability so the molecule has time to do its job; and penetrating the targeted organs and cells. We believe these challenges have led researchers to focus on other approaches to cancer therapeutics. Our strategy seeks to overcome these delivery challenges by repurposing a particle used in humans extensively for imaging purposes to now deliver synthetic RNA molecules (called oligonucleotides) to cancer cells.
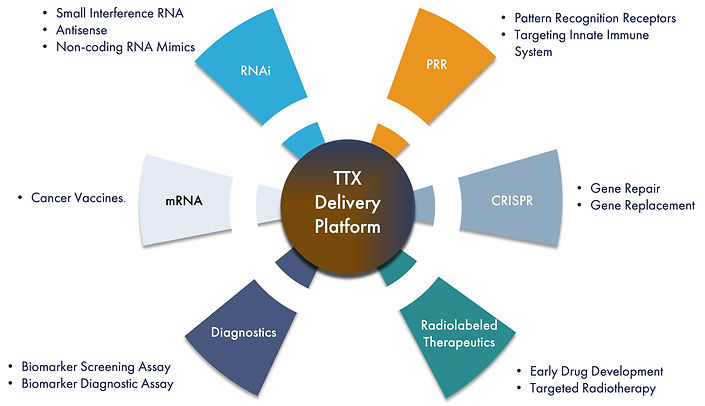
We utilize a modular drug design employing tools from the drug discovery toolbox we have created to develop product candidates that we believe can efficiently deliver RNA therapeutics to genetic targets. There are four distinct RNA strategies that we are developing to treat cancer including RNA interference, or RNAi, Pattern Recognition Receptors, or PRRs, Clustered Regularly Interspaced Palindromic Repeats, or CRISPR, and messenger RNA, or mRNA, vaccines. The first approach we are developing is using RNAi to “silence” or turn off the production of specific genes that cause cancer. RNAi is a natural biological process that regulates gene expression by “interfering” with mRNA, the carrier of DNA’s instructions for making new proteins. To date, we have utilized this approach to develop novel product candidates for treatment of metastasis and additional therapeutic areas in oncology. Our lead therapeutic candidate, TTX-MC138, is focused on treating metastatic cancer which has been shown to cause approximately 90% of all cancer deaths. TTX-MC138 targets microRNA-10b, or miR-10b, believed to be a critical driver of metastatic progression in a variety of solid tumors. We believe that TTX-MC138 has the potential to produce regression without recurrence in a range of cancers including breast, pancreatic, ovarian and colon cancer, glioblastomas and others. Our other RNAi therapeutic candidates are TTX-siPDL1 and TTX-siLIN28b which focus on treating cancer by targeting PD-L1, and Lin28b, respectively. The second RNA approach we are developing targets the retinoic acid-inducible gene I (RIG-I), a cytosolic nucleic acid sensing Pattern Recognition Receptor of the innate immune system. Our therapeutic candidate, TTX-RIGA, is intended to induce RIG-I activation , potentially provoking an immune response against cancer. We are conducting pre-clinical studies with TTX-SiPDL1, TTX-Lin28b and TTX-RIGA. Our very early discovery candidates, TTX-CRISPR and TTX-mRNA, are designed to treat cancer using the RNA approaches of CRISPR and mRNA vaccine technologies. All these therapeutic candidates are intended to utilize our proprietary delivery mechanism and are designed with the goal of significantly improving outcomes for cancer patients.
Additionally, we are pursuing diagnostic approaches for RNA targets that might be relevant and important to treating patients using RNA therapeutics. We have licensed a patented microRNA profiling assay with the potential to detect expression of microRNAs in patient blood and tissue samples. We intend to optimize this diagnostic test to detect miR-10b in cancer patients as our first commercial testing product. If approved, this test could be used as a screening assay to detect metastasis in a variety of tumor types. We may also be able to use it to evaluate miR-10b expression before, during and after treatment to best determine timing of therapeutic intervention.
Recent research published in Cancer Nanotechnology, entitled “Radiolabeling and PET-MRI microdosing of the experimental cancer therapeutic, MN-anti-miR10b, demonstrates delivery to metastatic lesions in a murine model of metastatic breast cancer.” This paper reported an MGH study using a radiolabeled derivative of TTX-MC138 (referred to in the paper as MN-anti-miR10b) tagged with Cu-64. As a result, highly sensitive and specific quantitative determination of pharmacokinetics and biodistribution, as well as observation of delivery of the Cu-64 labeled TTX-MC138 to metastases, was made using noninvasive PET-MRI. The key results of the study suggest that TTX-MC138, when injected intravenously, accumulates in metastatic lesions suggesting that our TTX platform delivers its therapeutic as intended. The MGH investigation describes a microdosing PET-MRI approach that can be used to measure TTX-MC138 biodistribution in cancer patients and its delivery to clinical metastases. The capacity to carry out microdosing PET-MRI studies in patients under an Exploratory Investigational New Drug, or eIND, application could be important because it has the potential to facilitate FDA authorization of initial human studies. This research, published by Dr. Medarova, our Chief Technology Officer and scientific co-founder, describes what we believe is a more effective approach to demonstrate delivery of TTX-MC138 in metastatic cancer patients. Since the PET technique is sensitive enough to determine the concentration of radiolabeled drug in the sub-picomolar range, microgram quantities of the radiolabeled drug are generally sufficient to perform such a study in humans. We believe this capability has significant advantages in the initial phases of drug development. Because the low mass of the radiolabeled drug does not induce reactions in humans, we believe the regulatory process will be less complex.
Due to the benefits we believe we can derive from a microdosing Phase 0 trial and enabled by the new studies described in Cancer Nanotechnology, we intend to pursue a microdosing Phase 0 trial for our First in Human (FIH) clinical study. We have targeted submission of the eIND for the microdosing study in the first half of 2022.
Success in the microdosing study could also validate delivery generally for our TTX pipeline which potentially opens-up additional relevant RNA targets that have been previously undruggable. Concurrent with the Phase 0 study, we expect to complete additional studies to support an IND for a Phase I clinical trial with TTX-MC138 and then file the IND in the second half of 2022.