
SIWA Therapeutics, Inc. to move forward toward filing an IND for pancreatic cancer after showing its SIWA318H monoclonal antibody promotes remission in a pancreatic cancer humanized mouse model
SIWA Therapeutics, Inc. (“SIWA”), a Chicago-based biopharmaceutical company, and Phoenix, AZ based Translational Genomics Research Institute (“TGEN”), recently presented study results at the September 29-30, 2021, AACR Virtual Conference Pancreatic Cancer showing that SIWA’s proprietary humanized monoclonal antibody, SIWA318H, is efficacious in an immune-humanized mouse xenograft model for pancreatic cancer. The study was conducted by TGEN pursuant to a SIWA-sponsored research agreement. SIWA indicated that it would be presenting additional data at the December 2021 MedInvest Oncology Conference.
Results of the pancreatic cancer study in humanized mice treated with SIWA318H included:
-
Both the SIWA318H high dose and low dose treatment groups showed significant anti-tumor activity in a PSN1 human pancreatic cancer mouse xenograft tumor model by significantly reducing tumor growth;
-
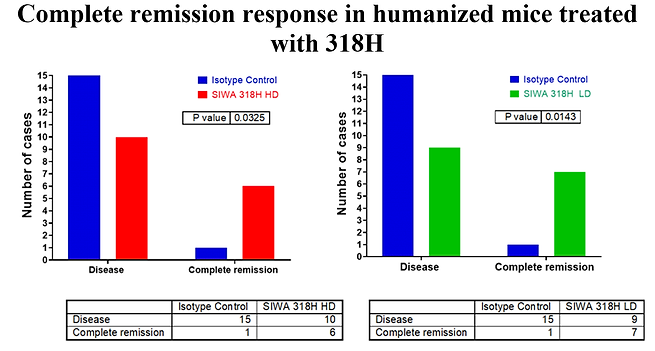
There was a significantly higher number of complete remission cases in the treated groups compared to the isotype-treated control group – see the graph below;
-
There was a trend to increased overall survival in the SIWA318H high dose and low dose treated groups compared to the untreated control group with median overall survival for control group at 26 days while median overall survival for SIWA318H treated mice was more than 45 days, which was the study termination date; all surviving mice were euthanized at that time;
-
After the treatments were stopped, tumor regrowth in the SIWA318H high dose and low dose treated groups were significantly slower than that in the control group with a statically significant difference in the complete remission (CR) response:
-
37.5% (6 out of 16 mice) treated with SIWA318H high dose had a complete remission response;
-
43.8% (7 out of 16 mice) treated with SIWA318H low dose had a complete remission response;
-
-
There were no significant differences in body weight changes between the control and treatment groups, indicating SIWA318H at the doses used were well-tolerated.
SIWA indicated that the efficacy of SIWA318H at both the high and low dose treatment levels, suggests that a reduced dose treatment might be used for the clinical development phase as it could positively impact, including, by way of example, treatment cost.
The study was conducted by TGEN pursuant to a SIWA-sponsored research agreement.
In other studies, SIWA318H has been demonstrated to selectively target a biomarker on cells exhibiting a combination of: (a) oxidative stress and (b) an abnormally high level of glycolysis (Warburg effect). Cells with the SIWA318H marker include specifically: (1) virtually all cancer cells, (2) senescent cells, and (3) microbially-infected cells (viruses, bacteria and at least some parasites).
SIWA indicated that it expects to file its first IND with the FDA in late 2022 for pancreatic cancer; it also expects to request that the FDA grant a FastTrack designation.
According to SIWA, the marker targeted by SIWA318H has been shown to be present on all cancers tested (including pancreatic cancer, glioblastomas, and lymphomas). Supporting in vivo studies with 318M, a mouse homolog of SIWA318H, statistically significantly reduced SCs and promoted muscle growth in normally aged very old mice, back to the level of young mice controls. 318M also statistically significantly inhibited cancer metastasis with trending toward reduced primary tumor growth in a mouse 4T1 triple negative breast cancer model. SIWA also completed a non-cGMP tolerability/pharmacokinetics study for SIWA138H in non-human primates to develop pharmacokinetic information for uses including dosing in humanized mice as well as ancillary support for future filing of an IND application with the FDA, As with the pancreatic cancer study, there were no significant differences in body weight changes between the control and treatment groups, indicating SIWA318H at the doses up to the 100 mg/kg used in the study were well-tolerated. SIWA indicated that the 318M and tolerability/pharmacokinetics studies were performed for it by Charles River Laboratories, Inc.
About SIWA: SIWA Therapeutics, Inc. is focused on cancers with a high unmet medical need and potential for FDA fast tracking/breakthrough designation (i.e., eligible for FDA Fast-tracking). SIWA318H selectively targets an advanced glycation end product (AGE) biomarker on cells exhibiting a combination of: (a) oxidative stress and (b) an abnormally high level of glycolysis (Warburg effect). Cells with this marker include specifically: (1) cancer cells, (2) senescent cells, and (3) microbially-infected cells. SIWA318H has been shown to bind to all three cell types, including cells infected by SARS-CoV-2 (COVID-19) and Influenza A. In 2020, SIWA was named a “key player” in Anti-Aging Drugs by the MIT Technology Review in its prestigious list of the 10 Breakthrough Technologies of 2020.
______________
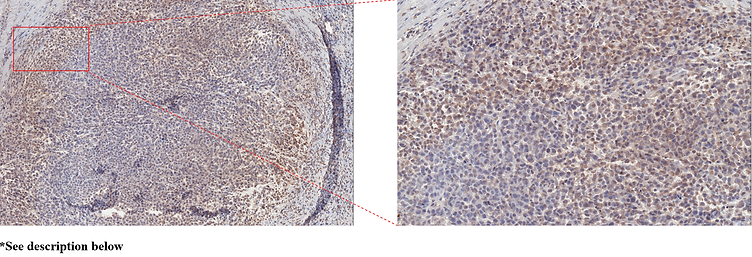
*The photomicrograph above shows binding of SIWA318H to PSN1 xenograft tumor cells; the cells were obtained from an orthotopic tumor that was harvested from a CD34+ humanized mouse. PSN1 is a cell line derived from a patient with Pancreatic Ductal Adenocarcinoma (PDAC). After staining, cancer cells were stained with peroxidase (brown) indicating binding to SIWA318H.