
SageMedic Aims to Bring Precision Medicine to All Cancer Patients
According to the National Cancer Institute’s MATCH trial and consistent with an analysis published in JAMA Oncology only a small fraction of cancer patients benefit from genome-driven targeted therapies[1] and even less from immunotherapies. A Redwood City-based private oncology diagnostic company overcomes those limitations with their functional precision medicine platform designed to help the underserved majority of patients.
Thus, even today the standard of care for most cancer patients are certain chemotherapies based on the particular tumor type. However, on average only 30% of patients have an “objective tumor response” and for some patients the tumor continues growing while the patient suffers from traditional therapy related side-effects. Often, a therapy, other than the standard of care, would have been the best treatment, but practicing oncologists currently have no means to know what treatment would work best for a particular patient – until now. Dr. Apfel, CEO of SageMedic said “It’s incomprehensible that cancer therapy is, for most parts, still hit or miss. Instead of trying it out on the patient, we should find the best treatment by directly testing the tumor so that the patient has a fighting chance to benefit from the therapy.”
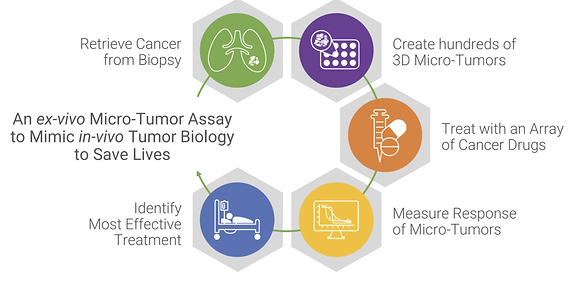
SageMedic has developed a proprietary, ex-vivo, high throughput assay, called the 3D SAGE Direct PlatformTM, that predicts patient-specific responses to various anti-cancer therapies, e.g. traditional cytotoxic and targeted therapies, and possibly also immunotherapies. The figure below, also on the company’s website, illustrates the processes. Their test takes the patient’s live tumor samples, to create hundreds of ex-vivo 3D micro-tumors that have similar heterogeneity and biology as the biopsy from the patient’s tumor, and then exposes those 3D micro-tumors to a dozen different treatment options and measures the responses. The treatment options can include chemotherapies, combinations of chemotherapies at differing doses, and even, combinations with precision therapies. Once SageMedic has correlated the ex-vivo responses with clinical outcomes, a sensitivity profile can be reported within a week to the treating oncologist to identify the most effective treatment for the patients.
[1] Marquart et al. JAMA Oncology 2018.
In addition to treating patients, the SAGE platform can be used in drug development. As the platform is cheaper, faster, and better than patient-derived xenograft (PDX) mice models, this is another revenue opportunity for the company by helping drug companies identify the most promising lead compounds before moving into the clinic. Undoubtedly, improving the success rate from Phase I to FDA approval from about 5% to 25% would not only reduce drug development risks but significantly increase the returns on R&D investments. More importantly, it could increase efficiency to bring novel treatments to market that would benefit cancer patients.
SageMedic’s primary business model is a fee-for-service model like Genomic Health, which is now a multi-billion-dollar company. Fresh, live tissue biopsy samples would be sent to SAGE overnight and the company would create a sensitivity profile for the treating oncologists within 1 week. At an MSRP of $3,600 per test, this is a $145M revenue opportunity (assuming 20% to 50% market penetration) for ovarian, breast, and colorectal cancer in the US alone. Furthermore, in a survey of 50 oncologists, over 91% would order the test right away if the test would lead to significantly improved patient outcomes and 67% would offer it to their patients even if there were no insurance coverage.
SageMedic (www.sagemedic.com) is now a CLIA-registered California laboratory and is starting their Series A capital raise to support pilot and cohort studies in ovarian, colon, and breast cancer patients within the next quarter.
Company Contact Information:
Chris Apfel, CEO
ph: 415-680-8266