Processa Pharmaceuticals Developing the Next Generation of Chemotherapy Drugs
Processa Pharmaceuticals, Inc. (NASDAQ: PCSA) is a clinical-stage pharmaceutical company with a mission to develop Next Generation Chemotherapy (NGC) drugs to improve the safety and efficacy of cancer chemotherapy treatment.
Processa’s novel NGC oncology drugs kill cancer cells with proven cancer-killing active molecules that are formed from some of the most widely used FDA-approved cancer drugs. The NGCs are designed to change the metabolism and/or distribution of the approved cancer drugs or their active metabolites while maintaining the mechanism of how the drug kills cancer cells. The Company’s present clinical and non-clinical evidence shows that its three NGC treatments (NGC-Cap, NGC-Gem, NGC-Irin) may provide an improved safety-efficacy profile when compared to their currently marketed counterparts - Capecitabine, Gemcitabine, and Irinotecan. Processa believes its NGC treatments have the potential to extend the survival and/or quality of life for more patients diagnosed with cancer while decreasing the number of patients who are required to dose-adjust or discontinue treatment because of side effects or lack of response.
The Company is developing these NGCs using its Regulatory Science Approach refined over the last 30 years. This Regulatory Science Approach has successfully demonstrated the positive benefit/risk relationship in more than 30 FDA approvals in indications across almost every division of the FDA. By combining these NGC treatments with its Regulatory Science Approach and experience using the principles of FDA’s recent Project Optimus Oncology Initiative, Processa is more likely to (i) determine an Optimal Dosage Regimen (ODR) based on the response-exposure relationship (as required by Project Optimus), (ii) obtain FDA approval, (iii) define a safety-efficacy profile better than their existing counterparts, and (iv) more efficiently develop each drug.
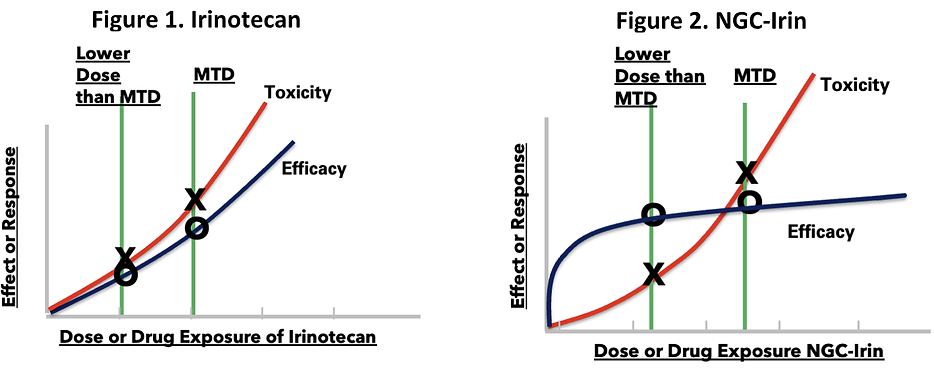
To better understand why FDA is requiring the use of its Project Optimus Oncology Initiative to determine and justify the ODR, one needs only to look at Processa’s mice study of Irinotecan (metabolized to the active molecule SN-38) vs Processa’s NGC-Irin (a novel pro-drug of SN-38) where the ODR for Irinotecan is the maximum tolerated dose (MTD),
while the NGC-Irin ODR is much less than its MTD. Figure 1 is a graph of the response vs dose of Irinotecan. Decreasing the dose of Irinotecan below the MTD decreases the severity and/or number of adverse events as shown by the red line but also decreases Irinotecan’s ability to inhibit tumor growth as shown by the blue line. Figure 2, however, shows that decreasing the dose of NGC-Irin below the MTD decreases the severity and/or number of adverse events but does NOT significantly change NGC-Irin’s ability to inhibit tumor growth, with almost 100% inhibition still occurring at doses much lower than the MTD. This data illustrates that the dose-response is dependent not only on the active cancer-killing molecule but also is dependent on the distribution and/or metabolism of the drug or its cancer-killing metabolite.
Processa’s goal is to determine the ODR for each NGC treatment while demonstrating the treatment is better for
patients than their current chemotherapy options.