
Featured Video Content
NORMAL AND NEOPLASTIC STEM CELLS
Dr. Irving Weissman, Director, Institute for Stem Cell Biology & Regenerative Medicine AND Stanford Ludwig Center for Cancer Stem Cell Research and Medicine
COVID: OUR LIFE SCIENCES SPUTNIK MOMENT
Dr. Ron DePinho, Immediate Past President, MD Anderson Cancer Center
TRANSFOMRATION IN CANCER DIAGNOSIS AND TREATMENT IN 2020
CMO, National Foundation for Cancer Research
Frm President, Emory & McGill Cancer Centers
Director, Darwin Institute
APPLYING FINANCIAL ENGINEERING TO CANCER
Dr. Andrew Lo, Director, Laboratory for Financial Engineering, MIT
PANEL: COVID'S AFFECTS ON CLINICAL TRIALS
Moderated By: Roy Herbst, Co-Head of Immunology at Yale Cancer Center
ENHCANCED DRUG DEVELOPMENT USING DRUG MECHANISM BIOMARKERS
Director DCTD Program Pharmacodyrnamic Biomarkers
REPURPOSING THE POLIO VACCINE IN THE WAR AGAINST COVID
Dr. Robert Gallo, Co-Founder and Director, The Institute of Human Virology (IHV) and Co-Founder HIV Virus
COVID: OUR LIFE SCIENCES SPUTNIK MOMENT
Dr. Jerome Kim, Director General of the International Vaccine Institute
BIOSECURITY: A MULTI-DIMENSIONAL CHALLENGE OF ESCALATING COMLEXITY AND URGENCY
George Poste, Professor, ASU; Former Head of R&D, GSK; Defense Science Board of the U.S. Department of Defense

MedInvest to Host Pharma and Biotech Investor Conference September 18-19, 2024
The upcoming Meeting of the MedInvest Pharma and Biotech Investor Conference will be hosted in New York City on September 18-19, 2024. The National Cancer Institute and O'Melveny Law Firm will be the Presenting Partners.
The event will feature keynote talks, panels, and corporate presentations from over 50 companies. Over 125 investors spanning venture capital, public funds, foundations, family offices and government grant making entities are expected to attend either virtually or in person.
IN THE WAR OF BEATING CANCER, STARPAX BIOPHARMA BRINGS A DISRUPTIVE TECHNOLOGY TO SOLVE A PROBLEM THAT HAS PERSISTED FOR OVER A CENTURY

Over the years magnificent and ingenious drug solutions to cure cancer were brought to the market by pharma companies. Today new discoveries still provide small steady incremental improvements in cancer treatments, but the overall response to cytotoxic chemotherapy seems to be unable to move higher than an average of 40%, with results for solid tumors ranging from 14% to 60% depending of the type of cancer (Maldonado, Parsons, Chen). Many immunotherapy studies show that approximately half of all human would not respond to target immunotherapy solutions. Because chemotherapy and immunotherapy appear to show resistance to efficacy, an important question needs to be addressed: Do these treatments reach their target? Over the years magnificent and ingenious drug solutions to cure cancer were brought to the market by pharma companies. Today new discoveries still provide small steady incremental improvements in cancer treatments, but the overall response to cytotoxic chemotherapy seems to be unable to move higher than an average of 40%, with results for solid tumors ranging from 14% to 60% depending of the type of cancer (Maldonado, Parsons, Chen). Many immunotherapy studies show that approximately half of all human would not respond to target immunotherapy solutions. Because chemotherapy and immunotherapy appear to show resistance to efficacy, an important question needs to be addressed: Do these treatments reach their target?

THE CASE FOR CONCARLO: CLEANING UP WHAT PRECISION ONCOLOGY LEAVES BEHIND
Advances in precision oncology have made the outlook for those with cancer more hopeful than ever. Through molecular profiling and genetic sequencing, medical professionals are now matching patients with the treatments that stand the highest likelihood of success. For example, if a woman has breast cancer that expresses high amounts of the estrogen receptor, she is a candidate for newer, more effectively targeted therapies such as the aromatase inhibitor Femara® or the estrogen receptor downgrader Falsodex®. Yet, despite this great progress, more than 600,000 patients continue dying each year in the U.S. from cancer. Sadly, even the most optimistic post-procedure patient meetings still end with an awkward discussion about the realities of what may have been left behind.

SIWA Therapeutics, Inc. to move forward toward filing an IND for pancreatic cancer after showing its SIWA318H monoclonal antibody promotes remission in a pancreatic cancer humanized mouse model
SIWA Therapeutics, Inc. (“SIWA”), a Chicago-based biopharmaceutical company, and Phoenix, AZ based Translational Genomics Research Institute (“TGEN”), recently presented study results at the September 29-30, 2021, AACR Virtual Conference in Pancreatic Cancer showing that SIWA’s proprietary humanized monoclonal antibody, SIWA318H, is efficacious in an immune-humanized mouse xenograft model for pancreatic cancer. The study was conducted by TGEN pursuant to a SIWA-sponsored research agreement. SIWA indicated that it would be presenting additional data at the December 2021 MedInvest Oncology Conference.

SageMedic Aims to Bring Precision Medicine to All Cancer Patients

According to the National Cancer Institute, only about 5% of cancer patients benefit from genome-driven targeted therapies and even less from immunotherapies. SageMedic, a Redwood City-based private oncology diagnostic company, has developed a genome-free, precision medicine assay designed to help the leftover majority of patients.
Today, patients are prescribed chemotherapies based on the standard of care for their particular tumor type. However, only 30% of patients have an “objective tumor response” and for some patients the tumor continues growing while the patient suffers from traditional therapy related side-effects. Often, a therapy other than the standard of care would have been the best treatment, but practicing oncologists currently have no means to know what treatment would work best for a particular patient – until SageMedic.
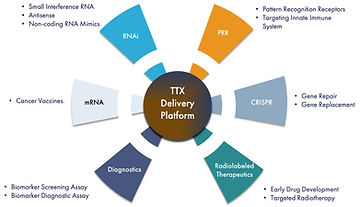
Delivering on the promise of RNA therapeutics to defeat cancer
TransCode Therapeutics is an RNA oncology company, created on the belief that cancer can be defeated through the intelligent design and effective delivery of RNA therapeutics.
For decades, RNA has been a topic of investigation by the scientific community as a potentially attractive therapeutic modality because it can target any gene and it lends itself to rational and straightforward drug design. RNA-based therapeutics are highly selective to their targets with the potential to make available a broad array of previously undruggable targets in the human genome. To date, use of RNA for therapy has been limited due to three delivery-related challenges: protecting the RNA from being dismantled by the immune system; maintaining stability so the molecule has time to do its job; and penetrating the targeted organs and cells.

OncoOne Advancing Novel Immunotherapies to Clinic Targeting the oxidized Macrophage migration Inhibitory Factor (oxMIF)
OncoOne, a Vienna, Austria based preclinical stage biotech company, has figured out how to drug an important yet “undruggable” target pivotal to our innate and adaptive immune response, the macrophage migration inhibitory factor, or MIF. MIF has been well characterized for over 50 years as a proinflammatory cytokine implicated in acute and chronic inflammatory conditions. In cancer, MIF is associated with high tumor burden, increased metastatic potential, and poor prognosis by driving pro-proliferative cell signaling, inhibiting cellular apoptosis and by exerting immunomodulatory activities in the tumor microenvironment which contribute to immune evasive and immune tolerant tumor phenotypes. OncoOne’s founders discovered that at sites of inflammation – such as an arthritic joint, and asthmatic lung, or a solid tumor -- MIF aggregates and undergoes a confirmational change, becoming the disease-related isoform of MIF, named by OncoOne’s founders oxidized MIF or oxMIF. By targeting oxMIF, OncoOne’s therapies counter regulate pro-tumorigenic and pro-inflammatory activities of MIF which will be tested clinically with the goal of improving patient outcomes.
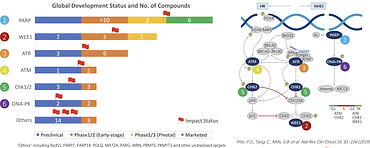
IMPACT Therapeutics: Building Leading Global Synthetic Lethality Product Pipeline for Next-Generation Anti-Cancer Targeted Therapy
Synthetic lethality is becoming an emerging approach for drug discovery and development recently. Genomic instability is one of the hallmarks of cancer cells, and the resultant mutations render cancer cells more dependent on alternative pathways for survival and proliferation, so these cancer cells are more vulnerable to targeted perturbations on those alternative pathways – a phenomenon known as synthetic lethality. Synthetic lethality can be exploited as next-generation targeted drug development approaches selectively target cancer cells with certain mutations, for example, deficiencies in DNA damage response (DDR).
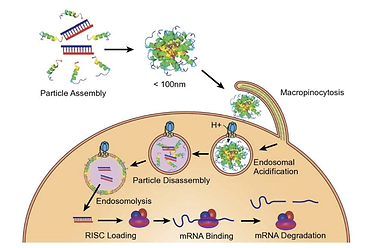
Altamira in strategic repositioning to develop RNA therapeutics
Altamira Therapeutics (NASDAQ:CYTO) is using its recent acquisition of closelyheld Trasir Therapeutics as a steppingstone for a strategic repositioning to focus on development of RNA therapeutics, while in the medium term aiming to spinoff or divest its legacy assets in neurotology, rhinology and allergology to unlock their value for the Company’s shareholders. “We aim to be a leading company for effective delivery of RNA payloads to tissues beyond the liver,” said chairman and CEO, Thomas Meyer, Ph.D.
As part of the repositioning, Altamira will become involved in oncology indications, which are particularly well suited for its RNA delivery technology. Dr. Meyer likened the potential of RNA therapeutics in oncology to the breakthroughs provided by monoclonal antibodies which started to make inroads from the 90s. In both cases, it took about 20 years between the actual innovation and the approval of the first drug. The first RNA therapeutic was approved by the FDA only in 2018 – hence the RNA breakthrough has now barely just got started.
TarMeta Biosciences: Combining Target With Phenotypic-Based Drug Discovery

TarMeta Biosciences, Inc. is a Boston based early stage drug discovery company with deep expertise in analytical chemistry whose mission is to identify and commercialize novel small molecule therapeutics for difficult to treat cancers. TarMeta’s drug discovery platform offers an approach to the drug discovery process that focuses on efficient and cost-effective pre-clinical drug development. By combining elements of both target and phenotypic based drug discovery as well as thermal shift assay, TarMeta’s platform allows for investigation of a molecule’s effects in a more physiologic environment and provides important information about the complex biological mechanisms that may be impacted upon treatment.

Felicitex Therapeutics has Designed First-In-Class Small Molecules that Inhibit DYRK Family Kinases to Selectively Disrupt Cancer Dormant Cells
Felicitex Therapeutics is an oncology drug development company in the Boston area. Our scientific platform is rooted in the biological systems that govern acquired resistance: specifically pathways that control cellular dormancy and are “hijacked” by cancer cells to enable their survival, dissemination throughout the body, and evasion of current standard-of-care therapies. Felicitex Therapeutics has designed first-in-class small molecules that inhibit DYRK family kinases to selectively disrupt cancer dormant cells in a manner that is specific to the disease and tissue context. This approach is lethal to cancer cells that rely on deregulation of the cell cycle for survival, and lead to reversal of acquired resistance mechanisms.
Finding Answers for Patients with Metastatic Cancer

Biocept (NASDAQ: BIOC) is one of the only molecular diagnostic companies focused on cerebrospinal fluid rather than blood. The company’s core technology looks at tumor cells in the cerebrospinal fluid, or CSF, as well as what's known as tumor-derived cell-free DNA, or tumor DNA. Biocept found that this combined approach is most informative, particularly when tumors have spread to the central nervous system.
